Discover The Hydrogen Bond Affinity Between Adenine And Thymine: Essential For Dna Stability And Function
How Many Hydrogen Bonds Between A and T?
Hydrogen bonds, crucial for DNA structure, form between specific base pairs. Adenine (A) and thymine (T), complementary bases in the Watson-Crick model, pair through two hydrogen bonds. Dipolar interactions between their electronegative N or O atoms and polarized H atoms create these bonds. The hydrogen bonds stabilize the A-T base pair, contributing to the stability and functionality of the double helix. Understanding these interactions is essential for DNA structure and function research.
The Intricate Dance of Hydrogen Bonds: Unraveling the DNA Code
In the realm of science, the discovery of hydrogen bonds unveiled a fundamental force shaping the very fabric of life. These bonds, like microscopic magnets, play a pivotal role in the structure and function of DNA, the blueprint of our genetic heritage.
Hydrogen Bonds: The Glue that Binds
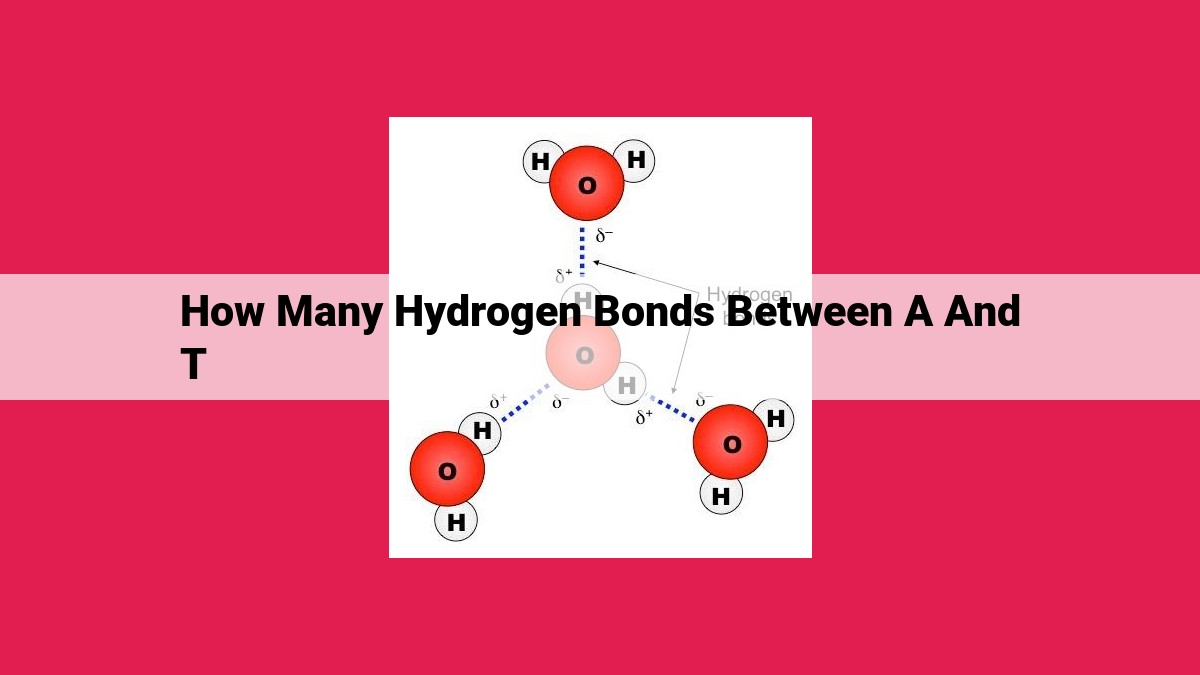
Imagine a molecular ballet, where atoms dance around each other. Hydrogen bonds are the invisible threads that connect these atoms, forming bridges between molecules. These bonds arise when a slightly positive hydrogen atom is attracted to a slightly negative atom, creating a dipole-dipole interaction.
In DNA, hydrogen bonds are the crucial force that holds the double helix together. They form between specific pairs of nitrogenous bases: adenine (A) with thymine (T) and guanine (G) with cytosine (C). This complementary base pairing is the foundation of DNA's structure, creating a twisted ladder-like arrangement.
The A-T Connection: A Duet
Of all the base pairs, the A-T bond is unique in its precisely determined number of hydrogen bonds. Unlike the G-C bond, which forms three hydrogen bonds, the A-T bond forms only two. This difference stems from the geometry of the molecules involved.
Adenine and thymine have specific chemical groups that form two dipole-dipole interactions. These interactions create two strong hydrogen bonds that stabilize the A-T base pair. This stability is further enhanced by van der Waals forces, which are weak attractive forces between molecules, and the hydrophobic effect, which favors the exclusion of water from nonpolar regions.
The Significance of Hydrogen Bonding in DNA
The number and strength of hydrogen bonds in DNA have profound implications for its structure and function. These bonds:
- Maintain the stability of the double helix, preventing it from unraveling.
- Facilitate DNA replication, allowing for the accurate copying of genetic information.
- Enable gene expression, where specific genes can be turned on or off by breaking and reforming hydrogen bonds.
Hydrogen bonds in DNA are not mere molecular connections; they are the lifeblood of genetic information. The precise number of two hydrogen bonds between adenine and thymine is a fundamental aspect of DNA's design, ensuring its stability and functionality. Understanding these interactions is crucial for unraveling the mysteries of life and unlocking the potential of genetic technologies.
Concepts Related to Hydrogen Bonding in DNA
Hydrogen bonds are crucial for the intricate architecture of DNA, playing a fundamental role in maintaining its iconic double helix structure. These bonds are formed due to dipolar interactions, arising from the unequal distribution of electrons within molecules. Dipolar molecules possess a positively charged end and a negatively charged end, allowing them to interact with each other through electrostatic attraction.
In the case of DNA, hydrogen bonds form between the nitrogenous bases, which include adenine (A), thymine (T), cytosine (C), and guanine (G). The specific pairing of A with T and C with G is known as complementary base pairing and is pivotal for DNA's stability and function.
Beyond dipolar interactions, van der Waals forces and the hydrophobic effect also contribute to hydrogen bond stability. Van der Waals forces arise from the temporary fluctuations in electron distribution, creating momentary electrical charges that induce weak attractive forces between molecules. The hydrophobic effect, on the other hand, refers to the tendency of nonpolar molecules (or parts of molecules) to aggregate in aqueous environments, forming a hydrophobic core. In the context of DNA, the hydrophobic effect stabilizes the hydrogen bonds between bases by shielding them from the surrounding water molecules.
Together, these forces orchestrate the formation and stabilization of hydrogen bonds in DNA, ensuring the proper pairing of bases and the maintenance of the double helix structure. This arrangement not only protects the genetic information stored within DNA but also facilitates its replication and expression, processes essential for life.
Complementary Base Pairing and the Watson-Crick Model:
- Introduce the concept of base pairing and highlight the specific pairing between adenine and thymine.
- Explain the significance of complementary base pairing for DNA structure and function.
- Discuss the Watson-Crick model and its description of the double helix structure.
Complementary Base Pairing and the Double Helix Model
In the intricate world of genetics, the blueprint of life, DNA, holds crucial secrets. Hydrogen bonds, the unsung heroes of DNA structure, play a vital role in shaping the genetic code. Among the four bases that make up DNA – adenine, thymine, cytosine, and guanine – the pairing of adenine and thymine stands out as a tale of perfect complementarity.
The discovery of complementary base pairing, a significant milestone in genetics, was made by the renowned scientists James Watson and Francis Crick. Their Watson-Crick model unveiled the double helix structure of DNA, forever transforming our understanding of genetic inheritance. According to this model, DNA consists of two antiparallel strands twisted together like a spiral staircase. The key to this structure lies in the specific pairing of bases: adenine (A) always pairs with thymine (T), and cytosine (C) with guanine (G).
This complementary pairing is driven by hydrogen bonds. Hydrogen bonds are intermolecular forces that form between electronegative atoms, such as nitrogen and oxygen, and hydrogen atoms. In DNA, the nitrogen atoms of adenine and thymine, and the oxygen atoms of cytosine and guanine, engage in hydrogen bonding. These bonds, though individually weak, collectively create a strong and stable structure that holds the DNA strands together.
The significance of complementary base pairing goes beyond mere structural stability. It also ensures the replication of DNA with remarkable accuracy. During replication, the DNA strands separate, and each strand serves as a template for the synthesis of a new complementary strand. The precision of this process relies on the specific pairing rules, ensuring that each new strand faithfully matches the original sequence.
In conclusion, complementary base pairing and the resulting hydrogen bonds are the cornerstones of DNA's structure and function. The perfect complementarity of adenine and thymine, orchestrated by the intricate dance of hydrogen bonds, not only maintains the stability of the DNA molecule but also ensures the faithful transmission of genetic information from one generation to the next.
Chargaff's Rules and the Significance of DNA Base Composition
Unraveling the Secrets of DNA's Architecture
In the realm of molecular biology, the enigmatic double helix of DNA stands as a marvel of nature's intricate design. Its structure, stability, and function hinge upon the precise arrangement of its constituent base pairs, each composed of a purine and a pyrimidine. Among these base pairs, the hydrogen bonds between adenine and thymine play a crucial role in shaping the DNA molecule's unique characteristics.
Chargaff's Rules: A Guiding Principle
In the early 1950s, Erwin Chargaff conducted groundbreaking research that revealed fundamental patterns in the base composition of DNA. His observations, known as Chargaff's rules, established that within a given species, the proportions of adenine and thymine are approximately equal, as are the proportions of guanine and cytosine.
Implications for DNA Structure and Stability
Chargaff's rules have profound implications for the structure and stability of DNA. They indicate that the base composition of DNA is not random but rather follows a specific pattern. This pattern arises from the complementary nature of base pairing, where adenine always pairs with thymine and guanine always pairs with cytosine.
Purine-Pyrimidine Balance: A Structural Keystone
The balance between purines (adenine and guanine) and pyrimidines (thymine and cytosine) is crucial for DNA's stability. The double helix structure is stabilized by the formation of hydrogen bonds between the complementary base pairs. The presence of equal amounts of purines and pyrimidines ensures that the number of hydrogen bonds formed between the two strands of the DNA double helix is consistent, contributing to its overall structural integrity.
Preserving the Genetic Blueprint
The precise arrangement of base pairs in DNA serves as the genetic blueprint for all living organisms. It contains the instructions for protein synthesis and other essential cellular processes. Chargaff's rules ensure that the base composition of DNA is conserved across generations, enabling the accurate transmission of genetic information during cell division.
Erwin Chargaff's discoveries unveiled the fundamental principles governing DNA base composition. His rules provide a deeper understanding of the structure and stability of the DNA double helix, highlighting the critical role of hydrogen bonding in preserving the integrity of our genetic heritage. Chargaff's legacy continues to inspire researchers today, who continue to explore the intricacies of DNA and its role in life's diverse processes.
The Two Hydrogen Bonds Between Adenine and Thymine: The Pillars of DNA Stability
In the captivating tale of DNA, the double helix, the blueprint of life, the hydrogen bonds between adenine and thymine play a pivotal role. These microscopic interactions weave together the genetic code, providing the foundation for the inheritance and expression of traits.
Dipole Dance: The Source of Attraction
Hydrogen bonds are electrostatic bonds that form when a hydrogen atom is sandwiched between two electronegative atoms, such as oxygen or nitrogen. In the case of adenine and thymine, the dipolar nature of these nitrogenous bases allows them to engage in a captivating dance of attraction.
The Hydrogen Bond Embrace
The amino group of adenine, with its slight positive charge (δ+), forms a hydrogen bond with the carbonyl group of thymine, which carries a slight negative charge (δ-). This dipole-dipole interaction creates a strong electrostatic attraction, drawing the two bases together like magnets.
A Second Embrace: Reinforcing the Bond
Not content with just one embrace, adenine and thymine engage in a second hydrogen bond. The amino group of thymine forms a hydrogen bond with the carbonyl group of adenine, further strengthening their bond. These complementary hydrogen bonds are the key to the stability of the adenine-thymine base pair.
Van der Waals and Hydrophobic Boosters
Beyond the dipole-dipole attraction, van der Waals forces also contribute to the stability of the adenine-thymine pair. These weak, attractive forces arise from the interactions between the electron clouds of neighboring atoms. Additionally, the hydrophobic effect helps to stabilize the hydrogen bonds by excluding water molecules from the hydrophobic core of the double helix.
The Impact of Hydrogen Bonds
The two hydrogen bonds between adenine and thymine are crucial for the structure and function of DNA. They provide the necessary stability for the double helix to maintain its shape, preventing the genetic code from becoming scrambled. Furthermore, they enable the accurate replication and transcription of genetic information, ensuring the faithful transmission of traits.
The two hydrogen bonds between adenine and thymine are the unsung heroes of the genetic world. They form the foundation for DNA stability, safeguarding the blueprint of life. Their story is a testament to the power of seemingly simple interactions, shaping the very fabric of our existence.
Related Topics:
- Correcting Lopsided Face: Understanding Causes And Treatment Options
- Optimized Seo Title:mitosis And Cytokinesis: The Key To Daughter Cell Creation
- Voltage-Gated Ion Channels: The Gatekeepers Of Cellular Communication
- Comprehensive Nail Cutter Maintenance Guide: Troubleshooting And Optimization
- Master The Pronunciation Of “Plumb”: Avoid Common Mistakes And Achieve Accurate Speech