Enthalpy Of Vaporization: Essential Calculations For Chemical Engineering
Enthalpy of vaporization, a critical property in chemical engineering, represents the heat energy required to transform a liquid into a vapor. To calculate it, experimental measurements or theoretical methods can be employed. One common approach is to use the Clausius-Clapeyron equation, which relates vapor pressure to temperature and enthalpy of vaporization. Alternatively, the Antoine equation enables vapor pressure estimation from temperature. Estimation methods like the Watson correlation and Joback method utilize critical temperature and molecular properties to approximate enthalpy of vaporization. These calculations find applications in process design, thermodynamic property determination, and predicting boiling point and vapor pressure behavior.
Enthalpy of Vaporization: The Key to Understanding Liquid-Gas Transitions
In the realm of chemical engineering and beyond, a profound concept known as enthalpy of vaporization holds the key to unlocking the mysteries of liquid-gas transitions. It's a tale of energy transformation, a dance between molecules where heat fuels their escape from liquid confinement into the ethereal realm of gas.
Enthalpy of vaporization, denoted by ΔHvap, represents the amount of heat energy required to convert a specific amount of a liquid into a gas at its boiling point and under constant pressure. This remarkable quantity holds immense significance, guiding engineers in optimizing processes, chemists in predicting thermodynamic behaviors, and scientists in unraveling the intricate world of molecular interactions.
Embarking on a Journey of Liquid-Gas Transformation
Envision a bustling hub of molecules within a liquid, their constant motion governed by the relentless laws of thermodynamics. As heat energy is poured into this liquid, its molecules gain kinetic energy, their vibrational dance becoming more vigorous. With each passing moment, a growing number of molecules reach a critical threshold where they overcome the attractive forces binding them to their liquid comrades. They break free, their ascent marked by the transition into the gaseous phase.
The Clausius-Clapeyron Equation: Unveilin
The relationship between enthalpy of vaporization and other fundamental thermodynamic properties is elegantly captured by the Clausius-Clapeyron equation. This mathematical masterpiece establishes a link between vapor pressure (P), temperature (T), and ΔHvap. It reveals that as temperature rises, so too does vapor pressure, and ΔHvap acts as the driving force behind this increase.
The Antoine Equation: A Convenient Tool for Vapor Pressure Estimation
In the realm of engineering and chemistry, the Antoine equation shines as a valuable tool for estimating vapor pressure from temperature. This empirical equation provides a convenient shortcut, allowing scientists to bypass the complexities of the Clausius-Clapeyron equation without compromising accuracy.
Watson Correlation and Joback Method: Predicting ΔHvap with Molecular Insights
Moving beyond empirical approaches, the Watson correlation and Joback method offer powerful tools for predicting ΔHvap based on molecular properties. These methods incorporate critical temperature and molecular weight (Watson correlation) or molecular structure (Joback method), providing valuable insights into the underlying molecular interactions that govern liquid-gas transitions.
Experimental and Theoretical Methods: Unveiling the Truth
Delving deeper into the realm of ΔHvap determination, both experimental and theoretical methods play pivotal roles. Experimental methods involve carefully measuring the heat required to vaporize a known liquid mass, providing highly accurate data. On the other hand, theoretical methods harness the power of equations and correlations, offering alternative pathways to ΔHvap estimation.
Applications: A Symphony of Engineering and Chemistry
The applications of enthalpy of vaporization span a vast array of industries and scientific disciplines. In process design, it serves as a cornerstone for optimizing distillation and evaporation processes, ensuring efficient energy utilization and maximizing product yield. In thermodynamic property calculations, ΔHvap enables the determination of entropy and Gibbs free energy, providing a comprehensive understanding of system behavior. Furthermore, its role in boiling point and vapor pressure estimation proves invaluable in various chemical and pharmaceutical processes.
Enthalpy of vaporization stands as a cornerstone of chemical engineering and other fields, offering a deep understanding of liquid-gas transitions and their profound implications. The concepts and methods outlined in this blog post provide a solid foundation for exploring this fascinating phenomenon, empowering engineers, chemists, and scientists alike to unlock the secrets of molecular transformations and harness their power for technological advancements and scientific breakthroughs.
Enthalpy of Vaporization: Unveiling the Secrets of Liquid-Gas Transformation
Picture this: you're peacefully enjoying a warm cup of coffee. As you gaze at its tranquil surface, you witness the subtle dance of vapor molecules, rising effortlessly into the air. This captivating process is driven by an invisible force known as enthalpy of vaporization. Join us as we delve into the fascinating world of this thermodynamic phenomenon.
Clausius-Clapeyron Equation: The Thermodynamics of Transformation
The Clausius-Clapeyron equation is an indispensable tool for understanding the relationship between vapor pressure, temperature, and enthalpy of vaporization. This equation elegantly describes how vapor pressure increases exponentially with temperature, a phenomenon we witness firsthand as water boils at 100°C.
Imagine a liquid trapped in a sealed container. As you heat the liquid, its molecules gain kinetic energy. Those with sufficient energy overcome the intermolecular forces holding them captive and escape into the gas phase. This process requires a certain amount of energy, known as enthalpy of vaporization.
The Clausius-Clapeyron equation provides a quantitative relationship between these variables:
ln(P) = -ΔHvap / (R * T) + C
where:
- P is the vapor pressure
- ΔHvap is the enthalpy of vaporization
- R is the ideal gas constant
- T is the temperature
- C is a constant
By manipulating this equation, we can calculate enthalpy of vaporization from experimental vapor pressure measurements or estimate vapor pressure from enthalpy of vaporization data.
Applications of Enthalpy of Vaporization: A Versatile Tool for Engineers
Enthalpy of vaporization is not just a theoretical concept; it has profound applications in various engineering disciplines.
- Process Design: Engineers rely on enthalpy of vaporization to design distillation and evaporation processes, ensuring efficient separation and purification of liquids.
- Thermodynamic Properties Calculation: Enthalpy of vaporization plays a crucial role in calculating other important thermodynamic properties, such as entropy and Gibbs free energy.
- Boiling Point and Vapor Pressure Estimation: Knowing the enthalpy of vaporization allows engineers to predict boiling points and vapor pressures, essential for designing and optimizing chemical processes.
Enthalpy of vaporization is an invaluable tool for chemical engineers, providing a comprehensive understanding of liquid-gas transformations. By harnessing its power, engineers can optimize processes, design more efficient systems, and ultimately advance the frontiers of chemical engineering.
The Antoine Equation: A Tool for Unveiling Vapor Pressure Secrets from Temperature
In the realm of thermodynamics, the Antoine Equation stands as a pivotal tool, empowering us to unravel the hidden connection between temperature and vapor pressure. This empirical equation, named after its ingenious inventor Charles Antoine, serves as a beacon for understanding the behavior of liquids in their gaseous state.
The Antoine Equation is a mathematical expression that encapsulates a nonlinear relationship between vapor pressure (P) and temperature (T). Its intricate formula unveils the manner in which P exponentially increases with rising T. This relationship holds true across a wide range of temperatures, allowing us to predict the vapor pressure of liquids under various conditions.
The equation takes the following form:
log(P) = A - B / (C + T)
where A, B, and C are constants specific to each substance. By determining these constants, we can precisely calculate the vapor pressure of a liquid at a given temperature.
The Antoine Equation finds its practical utility in numerous applications. For instance, it enables us to predict the boiling point of liquids - the temperature at which their vapor pressure equals atmospheric pressure. This knowledge is essential for industrial processes ranging from distillation to evaporation.
The equation also plays a crucial role in calculating thermodynamic properties, such as entropy and Gibbs free energy. These properties are indispensable for understanding the behavior of liquids in chemical reactions and other physical processes.
In conclusion, the Antoine Equation is an invaluable asset for deciphering the intricate relationship between vapor pressure and temperature. Its versatility extends across diverse applications, making it a cornerstone in the fields of chemical engineering and thermodynamics.
Delving into the Enthalpy of Vaporization: A Journey into Physics and Engineering
In the realm of chemical engineering, the enthalpy of vaporization plays a pivotal role. It represents the energy required to transform a liquid into its gaseous state. Understanding this concept is crucial for designing efficient processes and predicting various thermodynamic properties.
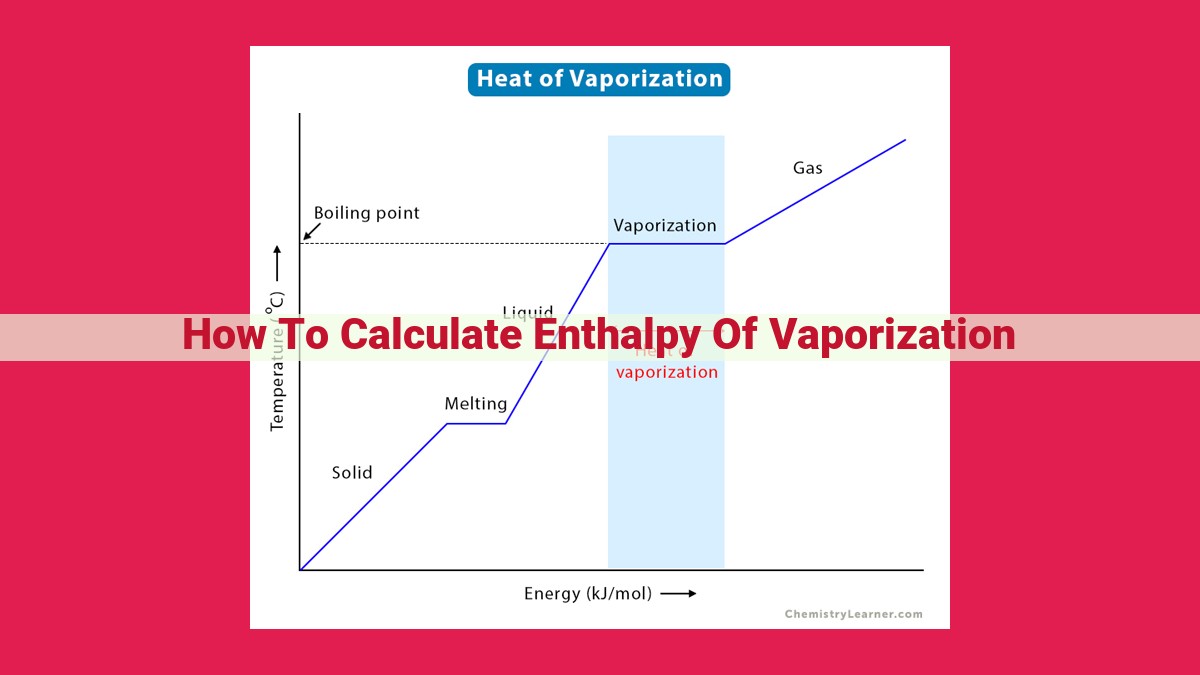
Imagine a pot of boiling water. As the liquid reaches its boiling point, it begins to vaporize, turning into steam. This transformation is not without energy consumption. The heat energy supplied to the water is used to break the intermolecular bonds holding the liquid molecules together, allowing them to escape into the gaseous phase. The enthalpy of vaporization quantifies the amount of energy required for this transition.
To calculate the enthalpy of vaporization experimentally, scientists employ calorimetry techniques. By measuring the heat required to vaporize a known mass of liquid, they can determine this critical property. However, theoretical methods also offer valuable insights.
One such method is the Watson Correlation. Developed by K.M. Watson, this correlation provides an empirical relationship between critical temperature, molecular weight, and enthalpy of vaporization. By utilizing this correlation, engineers can estimate the enthalpy of vaporization for various compounds without the need for extensive experimentation.
For instance, consider the compound n-hexane. Its critical temperature is 507.6 K, and its molecular weight is 86.18 g/mol. Using the Watson Correlation, we can estimate its enthalpy of vaporization as approximately 32.4 kJ/mol. This value can then be used in process design and thermodynamic calculations.
The Watson Correlation is a powerful tool for predicting enthalpy of vaporization. It simplifies the estimation process, enabling engineers to make informed decisions about process design and optimization. By understanding this concept and its practical applications, we can harness the power of thermodynamics to drive innovation in various industries.
Unlocking the Enthalpy of Vaporization: A Molecular Odyssey with the Joback Method
In the realm of chemical engineering and beyond, the enthalpy of vaporization holds a pivotal role, representing the energy required to transform a liquid into a vapor. Its profound impact extends across countless applications, from process design to thermodynamic calculations. Amidst the diverse methods for its determination, the Joback Method emerges as a powerful tool, harnessing the molecular structure of a substance to unveil its enthalpy of vaporization.
Delving into the Molecular Realm
The Joback Method recognizes the intrinsic relationship between a molecule's structure and its vaporization behavior. It ingeniously assigns specific molecular fragments numerical values, known as fragment constants. These constants capture the unique energy contributions of various molecular groups, such as carbon atoms, hydrogen atoms, and functional groups like alkenes and ketones.
Assembling the Enthalpy Puzzle
By piecing together the fragment constants, the Joback Method constructs a comprehensive molecular structure model. Each atom and functional group adds its energy contribution, like puzzle pieces fitting together. The sum of these contributions yields an accurate estimate of the total enthalpy of vaporization.
Simplicity and Versatility
The Joback Method stands out for its simplicity and versatility. Unlike other methods that require extensive experimental data or complex theoretical calculations, it requires only the molecular formula of the substance. This makes it an accessible and practical tool for a wide range of applications.
Applications Unbound
The applications of the Joback Method extend across multiple disciplines. In chemical process design, it aids in the design of distillation columns and evaporation processes. It also plays a crucial role in thermodynamic property calculations, providing insights into entropy and Gibbs free energy. Additionally, it enables accurate estimations of boiling points and vapor pressures, which are essential for predicting the behavior of liquids and vapors.
The Joback Method offers a remarkable approach to determining the enthalpy of vaporization, offering unparalleled simplicity and accuracy. By unraveling the molecular structure of a substance, it unlocks a wealth of information about its vaporization behavior. Its applications extend across a diverse range of fields, empowering engineers, scientists, and researchers to optimize processes, understand thermodynamics, and enhance their understanding of the molecular world.
Unveiling the Enigmatic Enthalpy of Vaporization
In the realm of chemical engineering and beyond, the enthalpy of vaporization emerges as a crucial concept. It unravels the hidden processes that drive a liquid's transition into its vaporous form. In this comprehensive guide, we will venture into the captivating world of enthalpy of vaporization, exploring its significance, essential concepts, and practical applications.
Understanding Enthalpy of Vaporization
The enthalpy of vaporization describes the amount of heat energy required to transform one mole of a liquid into a gas at its boiling point. It represents the energy needed to overcome intermolecular forces and create a new phase—the vapor phase. Enthalpy of vaporization holds immense value in various fields, such as chemical processing, power generation, and thermodynamics.
Essential Concepts
To delve deeper into the intricacies of enthalpy of vaporization, let's delve into some fundamental concepts:
- Clausius-Clapeyron Equation: This equation establishes a connection between vapor pressure, temperature, and enthalpy of vaporization, providing a powerful tool for understanding vapor-liquid equilibria.
- Antoine Equation: This empirical equation offers a convenient method to estimate vapor pressure from temperature, a crucial step in enthalpy of vaporization calculations.
- Watson Correlation and Joback Method: These methods leverage molecular properties, such as molecular weight and structure, to estimate the enthalpy of vaporization, extending its applicability to a diverse range of compounds.
- Other Related Terms: To fully grasp the landscape of enthalpy of vaporization, it's essential to define related terms such as latent heat of vaporization (energy released as a gas condenses), specific heat of vaporization (energy required to vaporize a unit mass of liquid), vapor pressure (pressure exerted by a vapor in equilibrium with its liquid phase), and boiling point (temperature at which vapor pressure equals ambient pressure, resulting in boiling).
Methods for Calculating Enthalpy of Vaporization
The quest to determine enthalpy of vaporization can be pursued through various avenues:
- Experimental Methods: These methods involve meticulously measuring the heat required to vaporize a known mass of liquid, providing highly accurate results.
- Theoretical Methods: Leveraging equations and correlations, these methods offer alternative approaches to calculating enthalpy of vaporization based on vapor pressure data, temperature dependencies, or molecular properties.
Applications of Enthalpy of Vaporization
The enthalpy of vaporization finds widespread application across industries, including:
- Process Design: It plays a pivotal role in designing distillation and evaporation processes, ensuring optimal performance.
- Thermodynamic Properties Calculation: It serves as a cornerstone for calculating other thermodynamic properties, such as entropy and Gibbs free energy.
- Boiling Point and Vapor Pressure Estimation: Enthalpy of vaporization enables precise predictions of these properties, which are crucial in various engineering applications.
The enthalpy of vaporization stands as an indispensable concept in thermodynamics and chemical engineering. By understanding its underlying mechanisms and essential concepts, we can unlock its potential in a wide array of practical applications. May this guide serve as a beacon of knowledge, illuminating the enigmatic world of enthalpy of vaporization.
Understanding Enthalpy of Vaporization
Enthalpy of vaporization is the amount of energy required to turn one mole of a liquid into a gas at a constant temperature and pressure. It's a crucial parameter in chemical engineering and other scientific disciplines.
Essential Concepts
- Clausius-Clapeyron Equation: This relationship between vapor pressure, temperature, and enthalpy of vaporization helps us calculate one from the others.
- Antoine Equation: This empirical equation estimates vapor pressure from temperature.
- Watson Correlation and Joback Method: These methods use molecular properties to estimate enthalpy of vaporization.
Methods for Calculating Enthalpy of Vaporization
Experimental Methods
The most direct way to measure enthalpy of vaporization is through calorimetry. In this technique, we heat a known mass of liquid in a sealed container until it completely vaporizes. The amount of heat required is the enthalpy of vaporization.
Calorimeters are carefully designed to minimize heat loss and precisely measure temperature changes. The liquid sample is placed in a sample pan and heated by a known amount of energy. As the liquid vaporizes, it absorbs energy, causing a temperature drop in the system. This temperature change is measured and used to calculate the enthalpy of vaporization.
Theoretical Methods
Besides experimental methods, we can also estimate enthalpy of vaporization using theoretical models. The Clausius-Clapeyron equation and the Antoine equation allow us to calculate enthalpy of vaporization from vapor pressure data. The Watson correlation and the Joback method use molecular structure to estimate enthalpy of vaporization.
Theoretical Methods for Calculating Enthalpy of Vaporization
Clausius-Clapeyron Equation
The Clausius-Clapeyron equation provides a theoretical approach to calculating enthalpy of vaporization using experimental vapor pressure data. This equation relates the vapor pressure (P), temperature (T), and enthalpy of vaporization (ΔHvap):
ln(P/P0) = -ΔHvap / (R * T) + C
where:
- P0 is the vapor pressure at a reference temperature
- R is the ideal gas constant
- C is an integration constant
By manipulating this equation and plotting ln(P/P0) versus 1/T, the slope of the line yields ΔHvap.
Antoine Equation
The Antoine equation is an empirical correlation that allows us to estimate vapor pressure from temperature. By combining this equation with the Clausius-Clapeyron equation, we can indirectly calculate enthalpy of vaporization:
P = exp(A - B/T - C/T^2)
where:
- A, B, and C are Antoine coefficients
By substituting the vapor pressure (P) obtained from the Antoine equation into the Clausius-Clapeyron equation, we can solve for ΔHvap.
Watson Correlation and Joback Method
These methods provide estimation techniques for enthalpy of vaporization using molecular properties. The Watson correlation relates ΔHvap to the critical temperature (Tc) and molecular weight (MW):
ΔHvap = 4.581 * Tc^0.35 * MW^0.85
The Joback method uses molecular structure to estimate ΔHvap. It considers various molecular fragments and their corresponding contributions to enthalpy of vaporization.
These theoretical methods offer valuable tools for estimating enthalpy of vaporization when experimental data is unavailable or challenging to obtain. They provide a convenient and reliable approach for engineering calculations and process design.
Calculating Enthalpy of Vaporization Using the Clausius-Clapeyron Equation
Unraveling the Mystery of Vaporization
Enthalpy of vaporization, a term often encountered in chemical engineering, refers to the amount of energy required to transform a liquid into a vapor at a constant temperature. It's a crucial concept in understanding the behavior of substances undergoing phase transitions.
The Equation that Unveils Enthalpy
The Clausius-Clapeyron equation, named after its inventors, provides a powerful tool for calculating enthalpy of vaporization. It establishes a relationship between vapor pressure, temperature, and enthalpy of vaporization.
$$ln(P) = -\frac{\Delta H_{vap}}{RT} + C$$
Where:
- P is the vapor pressure
- ΔHvap is the enthalpy of vaporization
- R is the universal gas constant
- T is the temperature
- C is an integration constant
Experimental Magic: Measuring Enthalpy
To utilize the Clausius-Clapeyron equation, we need experimental data on vapor pressure at various temperatures. Scientists employ sophisticated techniques to measure vapor pressure accurately, allowing them to construct a graph of ln(P) versus 1/T.
Slope to the Rescue
The slope of this graph, negative ΔHvap/R, provides the direct route to calculating enthalpy of vaporization. By measuring vapor pressures over a temperature range, we can experimentally determine this crucial thermodynamic property.
Insights from Vapor Pressure
The Clausius-Clapeyron equation sheds light on the relationship between enthalpy of vaporization and vapor pressure. As temperature increases, vapor pressure rises, while enthalpy of vaporization decreases. This inverse relationship underscores the fact that less energy is required to vaporize a substance at higher temperatures.
Handy Tool for Process Design and Beyond
The Clausius-Clapeyron equation is not just an academic curiosity but a practical tool used extensively in process design. Engineers calculate enthalpy of vaporization to optimize distillation and evaporation processes, ensuring efficient separation and purification of substances.
A Cornerstone of Thermodynamic Calculations
Enthalpy of vaporization is a cornerstone of thermodynamic calculations. Knowing this property allows us to estimate other thermodynamic properties, such as entropy and Gibbs free energy. It's a vital piece in the puzzle of understanding and manipulating the behavior of substances.
Antoine Equation: Unlocking Vapor Pressure and Enthalpy of Vaporization
The Antoine Equation is a powerful tool for estimating vapor pressure from temperature, which plays a crucial role in calculating enthalpy of vaporization. This equation provides a convenient and accurate way to predict the vapor pressure of a liquid at a given temperature.
The Antoine Equation takes the form:
log(P) = A - (B / (C + T))
where:
- P is the vapor pressure
- T is the temperature in Kelvin
- A, B, and C are constants
The constants A, B, and C are specific to each substance. They can be obtained from experimental data or from correlations based on molecular properties. By inputting the temperature of interest, the Antoine Equation can provide an estimate of the corresponding vapor pressure.
Knowing the vapor pressure, we can then use the Clausius-Clapeyron Equation to calculate the enthalpy of vaporization. This equation relates vapor pressure, temperature, and enthalpy of vaporization as follows:
(dP / dT) = (ΔHvap / (RT²))
where:
- dP / dT is the slope of the vapor pressure curve
- ΔHvap is the enthalpy of vaporization
- R is the ideal gas constant
- T is the temperature in Kelvin
By combining the Antoine Equation and the Clausius-Clapeyron Equation, we can establish a relationship between temperature and enthalpy of vaporization. This relationship allows us to estimate the enthalpy of vaporization based on vapor pressure data or temperature-dependent vapor pressure correlations.
The Antoine Equation is widely used in chemical engineering for process design, property estimation, and various other applications. Its ability to accurately predict vapor pressure makes it a valuable tool for understanding and controlling vaporization processes.
Enthalpy of Vaporization: A Comprehensive Guide for Chemical Engineers
Enthalpy of vaporization plays a pivotal role in chemical engineering and various other disciplines. It represents the energy required to convert a liquid into its vapor form at a constant temperature and pressure. Understanding this concept is crucial for designing efficient chemical processes.
The Clausius-Clapeyron equation establishes the relationship between vapor pressure, temperature, and enthalpy of vaporization. This equation provides a theoretical framework for calculating the enthalpy of vaporization from experimental vapor pressure data.
The Antoine equation offers an empirical approach to estimating vapor pressure from temperature. By incorporating the Antoine equation into the Clausius-Clapeyron equation, one can derive the enthalpy of vaporization.
Watson's correlation, a semi-empirical method, leverages critical properties and molecular weight for enthalpy of vaporization estimation. This method has proven particularly useful for nonpolar and slightly polar compounds.
Joback's method, on the other hand, utilizes molecular structure to predict enthalpy of vaporization. This approach requires detailed information about the molecular connectivity and functional groups present in the molecule.
These methods provide valuable tools for estimating enthalpy of vaporization in the absence of experimental data. They find applications in process design, thermodynamic property calculations, and predicting boiling points and vapor pressures.
Overall, the enthalpy of vaporization is a crucial parameter in chemical engineering and other disciplines. Its determination and estimation methods lay the foundation for efficient and accurate process design and analysis.
Enthalpy of Vaporization: A Key Factor in Process Design
When it comes to designing industrial processes involving distillation and evaporation, the enthalpy of vaporization plays a crucial role. It's a measure of the heat energy required to transform a liquid into a vapor at a constant temperature and pressure. Understanding this concept can help engineers optimize their designs for efficiency and productivity.
Distillation: Separating Components with Different Boiling Points
Distillation is a process in which a liquid mixture is heated to separate its components based on their different boiling points. The enthalpy of vaporization determines the amount of heat required to vaporize each component. By controlling the temperature and pressure during distillation, engineers can selectively vaporize and condense specific components, achieving the desired purity levels.
Evaporation: Concentrating Solutions and Purifying Water
Evaporators are used to concentrate solutions by removing water or other volatile liquids. The enthalpy of vaporization is essential in designing evaporators because it determines the amount of heat required to evaporate a given amount of liquid. Optimizing the heat input based on the enthalpy of vaporization helps minimize energy consumption and maximize evaporation efficiency.
Case Study: Designing a Distillation Column for Ethanol Production
In a typical ethanol production process, a distillation column is used to separate ethanol from water. The enthalpy of vaporization of ethanol is higher than that of water, meaning it requires more heat to vaporize. By adjusting the temperature and pressure within the column, engineers can control the vapor-liquid equilibrium and achieve the desired ethanol concentration in the distillate.
The enthalpy of vaporization is a critical parameter in process design, particularly in distillation and evaporation. By understanding its role, engineers can design processes that optimize energy efficiency, maximize productivity, and achieve desired product specifications. Its significance in process engineering cannot be overstated, contributing to advancements in industries ranging from pharmaceuticals to chemical manufacturing and water purification.
Thermodynamic Properties Calculation: Discuss the use of enthalpy of vaporization to calculate entropy and Gibbs free energy.
Enthalpy of Vaporization: A Key Thermodynamic Property for Engineering and Beyond
Understanding Enthalpy of Vaporization
Enthalpy of vaporization is a fundamental property that measures the energy required to transform a liquid into a gas. It's a crucial concept in engineering and chemistry, with applications ranging from process design to predicting the boiling points of substances.
Essential Concepts
- Clausius-Clapeyron Equation: Links vapor pressure, temperature, and enthalpy of vaporization.
- Antoine Equation: Empirical equation for calculating vapor pressure from temperature.
- Watson Correlation and Joback Method: Estimation methods for enthalpy of vaporization using molecular properties.
- Latent Heat of Vaporization: Energy required to overcome intermolecular forces during vaporization.
- Vapor Pressure: Pressure exerted by a vapor in equilibrium with its liquid or solid phase.
- Boiling Point: Temperature at which a liquid's vapor pressure equals the external pressure.
Methods for Calculation
Enthalpy of vaporization can be measured experimentally or estimated theoretically using methods like:
- Experimental Methods: Direct measurement of heat required to vaporize a known liquid mass.
- Clausius-Clapeyron Equation: Calculation using vapor pressure data.
- Antoine Equation and Correlation Methods: Estimation using molecular properties.
Applications
Enthalpy of vaporization plays a crucial role in various applications, including:
- Process Design: Optimizing distillation and evaporation processes in chemical plants.
- Thermodynamic Property Calculation: Determining entropy and Gibbs free energy.
- Boiling Point and Vapor Pressure Estimation: Predicting these properties for process control and safety considerations.
Enthalpy of vaporization is a critical thermodynamic property with wide-ranging applications. By understanding its concepts and calculation methods, engineers and scientists can harness its power to improve process efficiency, predict system behavior, and advance scientific knowledge.
Enthalpy of Vaporization: A Gateway to Predicting Boiling Point and Vapor Pressure
In the realm of chemistry and engineering, understanding the enthalpy of vaporization is crucial. It's the energy required to transform a liquid into a gas at a constant temperature. This concept holds immense significance in numerous applications, particularly in process design and thermodynamic calculations.
One of the most valuable applications of enthalpy of vaporization is in predicting boiling point and vapor pressure. The higher the enthalpy of vaporization of a substance, the stronger the intermolecular forces holding its molecules together. As a result, more energy is needed to break these forces and vaporize the liquid. This means substances with high enthalpy of vaporization have higher boiling points.
Similarly, vapor pressure is directly related to enthalpy of vaporization. The higher the enthalpy of vaporization, the lower the vapor pressure at a given temperature. This is because substances with high enthalpy of vaporization require more energy to escape into the gas phase, resulting in a lower concentration of vapor molecules in the air.
By accurately determining the enthalpy of vaporization, engineers and scientists can make precise predictions about the boiling point and vapor pressure of substances. This knowledge is essential for designing efficient distillation and evaporation processes, as well as for calculating other thermodynamic properties such as entropy and Gibbs free energy.
In summary, the enthalpy of vaporization serves as a key parameter in predicting the boiling point and vapor pressure of substances. Understanding this concept empowers us to control and manipulate these properties for various industrial and scientific applications.
A Comprehensive Guide to Enthalpy of Vaporization
In the realm of chemical engineering and other scientific disciplines, there exists a fundamental concept known as enthalpy of vaporization, a critical parameter that unveils the intricacies of liquid-to-vapor transformations. Understanding this phenomenon empowers us to tackle complex engineering challenges and unravel the secrets of thermodynamics.
Unveiling Enthalpy of Vaporization
Enthalpy of vaporization embodies the energy required to transform a liquid into a gas at constant temperature. Its enigmatic nature lies in its ability to provide insights into the intricate molecular interactions that govern phase transitions. This remarkable property plays a pivotal role in designing distillation and evaporation processes, guiding engineers in optimizing chemical separations and energy recovery systems.
Essential Concepts: Unraveling the Mysteries
Essential concepts intertwine to form the tapestry of enthalpy of vaporization. The Clausius-Clapeyron equation unveils the intimate relationship between vapor pressure, temperature, and enthalpy of vaporization. The Antoine equation empowers us to quantify vapor pressure from temperature data, a cornerstone in predicting liquid-vapor equilibrium.
The Watson correlation and the Joback method open the door to estimating enthalpy of vaporization using molecular properties. These methods harness the power of molecular structure to unravel the underlying energetic landscape of compounds. Furthermore, exploring latent heat of vaporization, specific heat of vaporization, vapor pressure, boiling point, and normal boiling point enriches our understanding of the interconnected thermodynamics of phase transformations.
Unleashing Methods for Calculation
An array of methods stands at our disposal for calculating enthalpy of vaporization. Experimental methods, the workhorses of thermodynamics, involve meticulously measuring the heat required to vaporize a known liquid mass. Theoretical methods offer an alternative path, leveraging vapor pressure data or molecular properties to estimate enthalpy of vaporization. These tools empower us to decipher the energetic landscape of liquids across a wide spectrum of conditions.
Applications: Engineering Marvels and Beyond
Enthalpy of vaporization extends its reach far beyond theoretical contemplation, finding applications in a myriad of engineering endeavors. Process design relies heavily on enthalpy of vaporization to optimize distillation and evaporation processes, unlocking the efficient separation of components. Thermodynamics revels in the versatility of enthalpy of vaporization, using it as a key ingredient in calculating entropy and Gibbs free energy. Predicting boiling point and vapor pressure, essential parameters in chemical engineering, becomes a reality with the guiding hand of enthalpy of vaporization.
This comprehensive journey through enthalpy of vaporization has unveiled its enigmatic nature, essential concepts, and practical applications. From understanding liquid-to-vapor transformations to mastering engineering processes, this fundamental property unravels the secrets of thermodynamics. As we continue to delve into the realm of energy transformations, enthalpy of vaporization will undoubtedly remain a beacon of knowledge, illuminating our path towards scientific advancements and engineering marvels.
Understanding Enthalpy of Vaporization: Its Importance and Applications
Enthalpy of vaporization, a fundamental concept in thermodynamics, quantifies the energy required to transform a liquid into a gas. This key parameter plays a pivotal role in numerous scientific and engineering disciplines.
Essential Concepts
The Clausius-Clapeyron Equation establishes the intricate relationship between vapor pressure, temperature, and enthalpy of vaporization. The Antoine Equation provides an empirical approach to calculate vapor pressure from temperature. Additionally, the Watson Correlation and Joback Method offer practical means to estimate enthalpy of vaporization based on critical properties or molecular structure.
Methods of Calculation
Experimental methods directly measure the heat needed to vaporize a known liquid mass. Theoretical methods utilize the Clausius-Clapeyron Equation, Antoine Equation, Watson Correlation, and Joback Method to calculate enthalpy of vaporization based on vapor pressure data or molecular properties.
Applications
Process Design: Enthalpy of vaporization is crucial in designing distillation and evaporation processes, as it determines the energy requirements and equipment specifications.
Thermodynamic Properties Calculation: It serves as a key parameter in calculating entropy and Gibbs free energy, providing a comprehensive understanding of thermodynamic systems.
Boiling Point and Vapor Pressure Estimation: Enthalpy of vaporization enables the accurate prediction and estimation of boiling points and vapor pressures, essential for process control and safety.
Enthalpy of vaporization is a fundamental property that governs the phase transition of liquids to gases. Its accurate determination and understanding are crucial for various applications in chemical engineering, thermodynamics, and other scientific and industrial fields. By delving into the concepts, methods, and applications of enthalpy of vaporization, we gain a deeper comprehension of the physical processes and energy transformations that shape our world.
Related Topics:
- Master The Pronunciation Of “Inebriation”: A Guide To Enhanced Communication
- Isolating The Variable N In Equations: A Guide To Solving For N
- Understanding The Longevity Of Dwarves: Factors Contributing To Their Remarkable Lifespans
- Cell Cycle Control: Essential For Health, Dysregulation Leads To Disease
- Unlocking The Illusion Of Depth: A Guide To Linear Perspective In Psychology