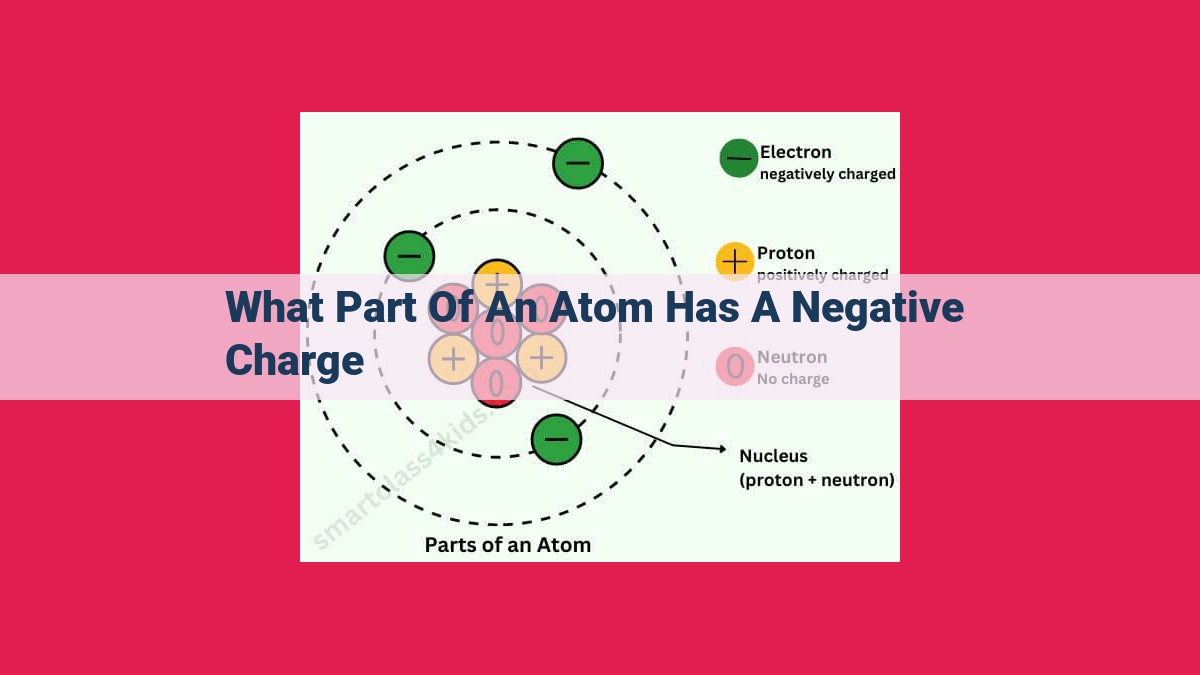
Electrons: Essential Subatomic Particles Driving Electrical Phenomena
Electrons, subatomic particles found in electron clouds outside the nucleus, carry a negative electric charge. These negative charges balance out the positive charge of protons within the nucleus, creating a neutral atom. Electrons are essential for the flow of electricity, as they carry electrical charges and contribute to electrical phenomena.
Electrons: The Fundamental Particles with a Negative Charge
In the realm of subatomic particles, electrons stand out as the tiny, negatively charged building blocks of matter. They play a pivotal role in shaping the world around us, from the flow of electricity to the formation of all known elements.
Understanding the Electron
At the heart of an atom, tiny electrons orbit the positively charged nucleus in a cloud-like region known as the electron cloud. These electrons possess an intrinsic negative electric charge, making them essential for balancing the positive charge of protons within the nucleus.
Electrons and Electricity
Electrons are the key players in the flow of electricity. When electrons move, they carry an electric charge. The flow of electrons through a conductor, such as a wire, creates an electric current, which po
Electrons and Magnetism
Moving electrons also generate magnetic fields. Imagine electrons spinning in loops like miniature magnets. As they spin, they create a magnetic field that interacts with other moving charges, giving rise to the fascinating phenomenon of magnetism.
The Significance of a Negative Charge
The negative charge of electrons plays a crucial role in the formation of atoms and molecules. Electrons are attracted to the positively charged protons in the nucleus, creating the building blocks of matter. The balance between positive and negative charges determines the chemical properties of elements.
Electrons and Energy
Electrons are not just passive particles; they also possess energy due to their movement. They can transfer or absorb energy in various processes, including chemical reactions and energy transformations. The energy levels of electrons are essential for understanding the behavior of atoms and molecules.
Electrons in the Fabric of Matter
Electrons are not isolated entities; they are fundamental building blocks of all matter. They contribute to the mass and chemical properties of atoms, shaping the structure of elements and compounds. Electrons are the glue that binds the universe together.
Electrons and Their Essential Role in the Flow of Electricity: A Story of Electric Currents
Imagine a microscopic world where tiny particles called electrons dance around atoms, carrying an electric charge that makes them essential players in the symphony of electricity. These electrons are the fundamental building blocks of electricity, the invisible force that powers our lives.
In the realm of electricity, electrons become the messengers of energy, carrying electric charges from one atom to another. When these charged electrons flow through a conductor like a wire, they create an electric current, the lifeblood of our electrical devices.
Electrons' ability to carry electric charges is what makes them so crucial in the world of electricity. They serve as the conduits, transporting electrical energy from power sources, like batteries and generators, to our homes, workplaces, and gadgets. Without these microscopic messengers, our modern world of lights, computers, and appliances would be plunged into darkness and silence.
Electricity is all about the movement of charged particles, and electrons play a starring role in this dance. Their inherent negative charge drives them to interact with other charged particles, creating the flow of electricity that powers our technological marvels. By understanding the role of electrons in electricity, we appreciate the unseen forces that shape our daily lives.
Electrons and Magnetism: The Invisible Force
Electrons, those tiny particles that whirl around the nucleus of an atom, do more than just carry an electric charge. They also possess an intriguing ability to create magnetic fields. Imagine electrons as miniature magnets, spinning in loops like tiny tornadoes. As they twirl, they generate an invisible force that we know as magnetism.
This magnetic field is like an invisible web that surrounds the moving electrons. It exerts a force on other nearby magnets and magnetic materials. This force can attract or repel, depending on the orientation of the magnets. For instance, when you bring two magnets together with their north poles facing each other, they push each other away due to the repulsive force generated by their magnetic fields.
The strength and direction of the magnetic field depend on the speed and number of electrons moving in the loop. Faster-moving electrons and more electrons result in a stronger magnetic field. This is why some materials, such as iron and nickel, are more magnetic than others like wood or plastic. Iron and nickel have a higher number of electrons that can move freely and form loops, creating a more robust magnetic field.
The relationship between electron movement and magnetism is a fundamental principle in electromagnetism. It forms the basis of various electrical devices, from electric motors to MRI machines. In an electric motor, for example, the spinning of electrons in loops creates a magnetic field that interacts with a permanent magnet. This interaction generates a force that causes the motor to rotate.
Understanding the magnetic properties of electrons is crucial for numerous applications in science and technology. It enables us to harness the power of magnetism for practical purposes, from generating electricity to studying the human body. As we delve deeper into the world of magnetism, we continue to uncover the remarkable abilities of these tiny, spinning electrons.
The Negative Charge of Electrons: An Essential Aspect of Matter
Electrons, the cornerstone of our physical world, possess a fundamental property that shapes their behavior and the very nature of matter itself: their negative electric charge. This attribute sets them apart from their positively charged counterparts in the atomic nucleus, protons, and plays a crucial role in the formation of atoms, molecules, and the intricate tapestry of chemical reactions that govern our lives.
The negative charge of electrons is a fundamental attribute that distinguishes them from other particles. Unlike protons, which carry a positive charge, electrons possess an equal and opposite negative charge. This intrinsic characteristic determines their behavior in electric fields and their interactions with other charged particles.
The significance of the negative charge of electrons is evident in the formation of atoms. Atoms, the basic building blocks of all matter, consist of a central nucleus containing protons and neutrons, surrounded by a cloud of orbiting electrons. The positive charge of the protons attracts the negatively charged electrons, binding them together and creating the stable structure of an atom. The negative charge of electrons also governs the chemical properties of atoms, determining their ability to form bonds with other atoms and participate in chemical reactions.
The negative charge of electrons plays a vital role in the formation of molecules, the building blocks of all matter we see around us. When atoms come together to form molecules, they share or exchange electrons. The negative charge of electrons determines the nature of the chemical bonds that form between atoms, giving rise to the diverse array of molecules that make up our world.
In conclusion, the negative charge of electrons is a fundamental aspect of matter, shaping the structure of atoms, molecules, and the interactions between charged particles. It underlies the very fabric of our physical world and is essential for understanding the myriad phenomena that govern our daily lives. From the flow of electricity to the chemical reactions that sustain life, the negative charge of electrons plays a pivotal role in the intricate tapestry of the universe.
Electrons and Energy: The Powerhouse of the Universe
In the realm of physics, electrons reign as fundamental building blocks of matter, imbued with a unique negative electric charge that shapes their behavior and the world around us. These subatomic particles play a pivotal role in electricity, magnetism, and the intricate dance of energy transformations that drive our universe.
Electrons as Energy Carriers
Imagine electrons as tiny energy capsules. Their ceaseless motion endows them with an inherent energy that manifests in various forms. Like restless travelers, electrons are constantly exchanging this energy, weaving a complex tapestry of interactions. They dance through electrical circuits, carrying electric charges like invisible couriers, enabling the flow of electricity that powers our devices.
Energy Exchange and Chemical Reactions
Beyond their role in electricity, electrons also play a crucial part in chemical reactions. Think of them as chemical messengers, facilitating the transfer of energy between atoms. In the intricate ballet of chemical reactions, electrons can donate or accept energy, leading to the formation and breaking of chemical bonds. They orchestrate the creation of molecules, the very building blocks of all living organisms.
Energy Transformations and Life's Processes
The energy possessed by electrons extends beyond chemical reactions. It drives countless biological processes that sustain life itself. In photosynthesis, the energy of sunlight is captured and stored by electrons, providing plants with the fuel they need to thrive. Moreover, the energy of electrons powers the metabolic processes that enable living beings to function, from muscle movement to brain activity.
Understanding Electrons: A Key to Unlocking the Universe
By unraveling the mysteries of electrons, we gain a profound understanding of the fundamental forces that shape our world. From the flow of electricity to the intricacies of life's processes, electrons are the puppet masters behind the scenes. As we delve deeper into their realm, we unlock the secrets of energy transformations and shed light on the enigmatic dance of the universe.
Electrons: The Fundamental Building Blocks of Matter
In the tapestry of the universe, where matter weaves its intricate dance, electrons occupy a place of profound importance. These subatomic particles, invisible to the naked eye yet imperative for life's existence, are the cornerstone of our physical reality.
Electrons as Building Blocks
Imagine a vast, boundless expanse of atoms, each a tiny microcosm of its own. Within these atoms lies a microscopic realm where electrons reside, orbiting the nucleus like a celestial ballet. These negatively charged particles are the fundamental building blocks of matter, the essence that gives physical form to everything around us.
Mass and Chemical Properties
While electrons are insignificant in mass compared to protons and neutrons, they play a crucial role in determining an atom's chemical properties. By interacting with other electrons, they dictate the number and arrangement of chemical bonds, shaping an atom's ability to combine with other elements and form molecules.
Formation of Elements and Compounds
The negative charge of electrons makes them attracted to the positively charged protons in atomic nuclei, forming the stable structures that comprise elements. As atoms interact with each other, their electrons can be transferred or shared, creating diverse chemical bonds. These bonds determine the unique properties of compounds, from the hard, brittle nature of salt to the soft, pliable texture of rubber.
In conclusion, electrons, though minuscule, are the very essence of matter. They contribute to its mass, shape its chemical properties, and facilitate the formation of elements and compounds. Without these fundamental building blocks, the universe as we know it would be a vast, empty void.
Related Topics:
- Watersense: Your Guide To Water Conservation And Savings
- Shared Features Of Chloroplasts And Mitochondria: Key Characteristics
- Understanding Age: Importance In Personal, Legal, And Cultural Contexts
- Uv Light Protection: The Role Of Melanin And Other Pigments
- The Ultimate Guide To Savoring The Art Of Swisher Sweet Cigars