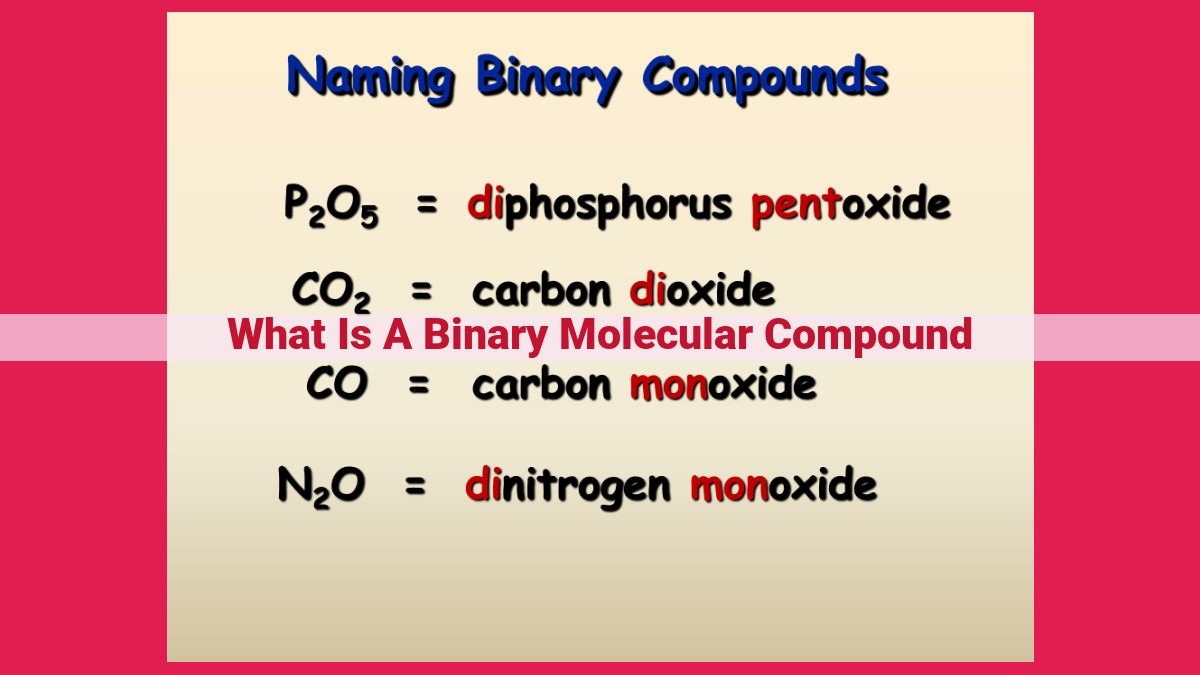
Binary Molecular Compounds: A Comprehensive Understanding For Chemists
Binary molecular compounds are chemical compounds consisting of two nonmetallic elements that form molecules via covalent bonds. Nonmetals, located on the right side of the periodic table, tend to have multiple valence electrons and react by sharing them to achieve stability. In binary molecular compounds, these shared electrons form covalent bonds between the nonmetal atoms, creating a stable molecule. Lewis structures, which depict the arrangement of electrons and bonds, are used to represent these compounds graphically. Binary molecular compounds exhibit unique properties and characteristics, making them essential in various fields of chemistry.
Demystifying Binary Molecular Compounds: A Journey into the Realm of Elements and Bonds
In the vast tapestry of chemical compounds, binary molecular compounds stand out as a fascinating group that captivates the imagination. These substances, formed by the union of two elements, reveal intriguing properties that unravel the secrets of atomic interactions.
Step into a molecular world where electrons dance and atoms unite to create remarkable compounds. Binary molecular compounds are composed of two nonmetal elements, which reside in the upper right-hand corner of the periodic table. These elements share a common characteristic: their insatiable desire for electrons.
Their quest for electron sharing fuels the formation of covalent bonds, the backbone of binary molecular compounds. In a covalent bond, electrons are not transferred but rather shared between atoms, creating a molecular union that holds them together like a chemical embrace. The resulting molecule, a stable entity composed of two or more atoms, becomes a new building block in the world of chemistry.
Nonmetals, with their eagerness to share electrons and form covalent bonds, become the architects of binary molecular compounds. Their location in the periodic table, near the cusp of reactivity, makes them prime candidates for these chemical alliances.
**Elements: The Building Blocks of Binary Molecular Compounds**
In the realm of chemistry, binary molecular compounds reign supreme, captivating the minds of scientists with their intriguing composition. At their core lie nonmetals, the key players that form these compounds through a fascinating dance of electrons. Nonmetals reside on the right side of the periodic table, boasting a remarkable ability to share elec
Imagine a row filled with these nonmetal elements, each with its own unique personality. They're like shy teenagers at a school dance, eager to socialize but also a bit hesitant. Hydrogen, the cool kid on the block, is always ready to party with its one lonely electron. Oxygen, the glamorous diva, has six electrons to flaunt, attracting attention from all around. Nitrogen, the reserved artist, prefers to keep four electrons to itself. Carbon, the versatile chameleon, can mingle with a wide range of electron partners.
Nonmetals possess a special trait that makes them the perfect candidates for forming binary molecular compounds: their high electronegativity. Electronegativity measures an element's ability to attract electrons. Nonmetals have a strong urge to pull electrons towards themselves, making them willing participants in the electron-sharing game.
This electron-sharing dance is what gives rise to covalent bonds. In a covalent bond, two nonmetal atoms join forces, sharing electrons to create a stable and mutually beneficial partnership. It's like two dance partners twirling around the dance floor, each contributing their own energy to the harmonious movement.
Covalent Bonds: The Binding Force of Binary Molecular Compounds
In the fascinating world of chemistry, we encounter binary molecular compounds—chemical entities composed of two different nonmetallic elements. Understanding the nature of these compounds requires delving into the intricate world of covalent bonds, the invisible forces that hold their atoms together.
Covalent bonds are formed when nonmetals, elements located on the right-hand side of the periodic table, share their electrons. Unlike ionic bonds, where electrons are transferred from one atom to another, covalent bonds involve the mutual sharing of electrons.
These shared electrons form electron clouds that surround the nuclei of the bonded atoms. The covalent bond entsteht between the two atoms, representing the region where their electron clouds overlap. The strength of the covalent bond depends on the number of shared electrons and the overlap of their electron clouds.
In binary molecular compounds, each atom "wants" to achieve a stable electron configuration, typically with eight valence electrons. Through covalent bonding, they can achieve this stability by sharing electrons to complete their valence shells. The result is a stable molecule, where the shared electrons "belong" to both atoms involved in the bond.
Covalent bonds offer significant advantages over other types of chemical bonds. They allow for the formation of a wide variety of molecular shapes and structures, from simple diatomic molecules to complex organic compounds. Covalent bonding is also responsible for the unique properties of many materials, such as plastics, fabrics, and even the DNA that forms the blueprint of life.
Molecules: The Building Blocks of Binary Molecular Compounds
In the vast expanse of chemistry, binary molecular compounds play a pivotal role in shaping the world around us. These compounds are so named because they are composed of just two elements, and they share a special bond known as a covalent bond.
A molecule is a group of atoms that are held together by covalent bonds. Covalent bonds occur when atoms share electrons in order to achieve a more stable electron configuration. In binary molecular compounds, the atoms involved are typically nonmetals.
Nonmetals are elements that are located on the right-hand side of the periodic table. They are generally poor conductors of heat and electricity, and they tend to be brittle and reactive. When two nonmetals combine, they form a binary molecular compound.
The formation of a molecule can be depicted using a Lewis structure. A Lewis structure is a diagram that shows the arrangement of atoms and electrons in a molecule. In a Lewis structure, the atoms are represented by their element symbols, and the electrons are represented by dots.
For example, consider the binary molecular compound hydrogen chloride (HCl). The Lewis structure for HCl is:
H:Cl
In this Lewis structure, the hydrogen atom is represented by the symbol "H", and the chlorine atom is represented by the symbol "Cl". The dot between the hydrogen and chlorine atoms represents the covalent bond that holds them together.
Molecules are the basic building blocks of matter. They are responsible for the properties of substances, and they play a vital role in chemical reactions. By understanding the nature of molecules, we can better understand the world around us.
Nonmetals: The Building Blocks of Binary Molecular Compounds
Nestled amidst the periodic table's vibrant hues lies a realm of elements that hold the key to unlocking the secrets of binary molecular compounds. These nonmetals are the architects of molecules, the fundamental units that govern countless chemical reactions and shape the very fabric of our world.
In the periodic table's upper right corner, these nonmetallic elements dance across groups 14-18, their characteristics as diverse as their presence. They lack the shiny luster of metals, instead donning dull or colorful appearances. These elements refuse to conduct electricity or heat, instead acting as electrical insulators.
Their chemical nature is equally distinct. Nonmetals exhibit an intense affinity for electrons, eagerly forming covalent bonds with other nonmetals. These strong bonds, created by the sharing of electrons, give rise to the molecular nature of binary molecular compounds.
Moreover, nonmetals are found high in the periodic table, near the halogens. The halogens, with their strong electronegativity, excel in attracting electrons from other atoms. This tendency explains their reactivity and their tendency to form bonds with less electronegative elements.
So, let us explore the captivating world of binary molecular compounds, where nonmetals reign supreme, orchestrating the formation of these essential chemical building blocks that orchestrate the myriad reactions that define our world.
Understanding Lewis Structures: A Visual Representation of Covalent Bonds
Binary molecular compounds, composed of two elements, exist due to the attractive forces between positively and negatively charged ions. The formation of these compounds is facilitated by covalent bonds, a type of chemical bond where atoms share electrons to achieve a more stable electron configuration. Lewis structures, a powerful tool in chemistry, provide a visual representation of these covalent bonds and electron distribution.
Delving into the World of Lewis Structures:
A Lewis structure is a diagram that represents the sharing of electrons between atoms in a molecule. It depicts the arrangement of atoms and the distribution of valence electrons, which are the electrons in the outermost shell of an atom. Valence electrons participate in chemical reactions by forming bonds with other atoms.
Constructing Lewis Structures:
To construct a Lewis structure, follow these steps:
- Determine the total number of valence electrons for all the atoms in the molecule.
- Connect the atoms with single bonds (each single bond represents two shared electrons).
- Distribute the remaining valence electrons as lone pairs (unpaired electrons) around the atoms, starting with the more electronegative atoms (those with a higher attraction for electrons).
- Check if the octet rule is satisfied for each atom. The octet rule states that an atom tends to achieve a stable electron configuration by sharing electrons to complete eight valence electrons.
Benefits of Lewis Structures:
Lewis structures are a valuable tool for understanding molecular structure and bonding. They provide insights into:
- The arrangement of atoms within a molecule
- The presence and nature of covalent bonds
- The distribution of electron density
- Molecular geometry and polarity
Lewis structures are a visual language that reveals the intricate world of covalent bonds and electron distribution in binary molecular compounds. By understanding Lewis structures, we gain a deeper appreciation of the chemical interactions that shape the molecular realm. These diagrams serve as a powerful tool for predicting molecular behavior and properties, opening the door to further exploration in the field of chemistry.
Related Topics:
- Uncover Protein Movements And Modifications: A Guide To Pulse-Chase Experiments
- Distilled Water: Understanding Neutral Ph And Autoionization
- Freezing Point Depression And Boiling Point Elevation: Effects Of Dissolved Substances On Water
- Maximize The Lifespan Of Your Cows: A Comprehensive Guide To Longevity
- Kratom Withdrawal: Timeline, Symptoms, Treatment, And Long-Term Effects