Understanding The Atomic Nucleus: The Building Blocks Of Elements
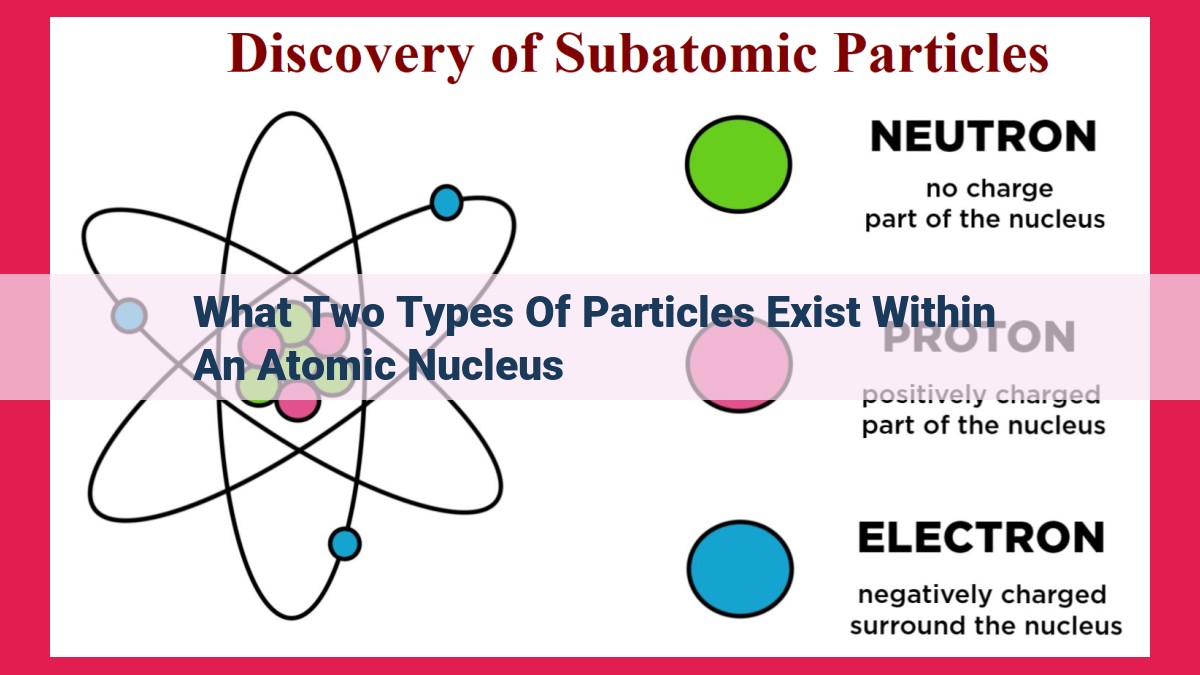
The atomic nucleus, the central core of an atom, houses two types of particles: protons and neutrons. Protons, positively charged, determine the element's identity and atomic number, while neutrons, neutral, contribute to nuclear stability. These particles interact through the strong nuclear force, which balances electrostatic repulsion, maintaining the integrity of the nucleus. The interplay of protons and neutrons influences atomic mass, nuclear stability, and behavior in chemical reactions, driving ongoing research in nuclear physics and practical applications such as nuclear energy and medical imaging.
Delving into the Heart of Matter: Unraveling the Secrets of Nuclear Particles
At the heart of every atom lies a tiny, dense core known as the nucleus. This enigmatic realm holds the protons and neutrons, fundamental particles that shape the very essence of matter. Embark on an atomic adventure as we uncover the secrets of these nuclear dwellers and their profound impact on the world around us.
Protons: The Positively Charged Guardians
Protons are the positively charged inhabitants of the nucleus, each carrying a unit of positive electrical charge. They determine the atomic number of an element, which defines its identity on the periodic table. The number of protons also dictates the strength of the electrostatic repulsion within the nucleus, a force that would otherwise tear atoms apart.
Neutrons: The Neutral Stabilizers
Neutrons, as their name suggests, are neutral particles with no electrical charge. Their role in the nucleus is crucial: they act as stabilizing forces, countering the electrostatic repulsion between protons. The number of neutrons in an atom can vary, giving rise to different isotopes of the same element. Isotopes have the same number of protons but differ in neutron count, affecting thei
Balancing Act: The Strong Nuclear Force
The nucleus is a delicate balancing act, where the electrostatic repulsion between protons threatens to tear it apart. Enter the strong nuclear force, a powerful force that overcomes electrostatic repulsion and binds protons and neutrons together. This intricate interplay of forces maintains the integrity of the nucleus, the very core of our atomic existence.
Imprints on the Atomic World
The number of protons and neutrons within an atom profoundly influences its atomic mass. Elements with more protons and neutrons are heavier than those with fewer. Moreover, the neutron-to-proton ratio affects nuclear stability and radioactivity. Radioactive elements have an unstable ratio, leading to the emission of particles and energy. These properties govern the behavior of elements in chemical reactions, shaping the molecular tapestry of the world.
Ongoing Explorations and Practical Applications
The realm of nuclear particles continues to captivate scientists, with ongoing research shedding light on their intricate nature and potential applications. Advances in nuclear physics pave the way for breakthroughs in nuclear energy, harnessing the power of nuclear reactions for sustainable electricity generation. Nuclear particles also play a vital role in medical imaging, enabling doctors to diagnose and treat diseases with unprecedented precision.
Protons: The Positively Charged Nucleus Backbone
At the heart of every atom lies the nucleus, a tiny, dense core packed with protons and neutrons. Protons, the positively charged members of this nuclear duo, play a crucial role in shaping the identity and properties of elements.
Defining the Atomic Number and Element Identity
Each proton carries a single positive charge, the fundamental unit of electricity. The number of protons in an atom's nucleus determines its atomic number, a unique identifier that distinguishes one element from another. For instance, hydrogen has one proton (atomic number 1), helium has two (atomic number 2), and oxygen has eight (atomic number 8).
The Strong Nuclear Force: Overcoming Repulsion
Despite their positive charges, protons coexist peacefully within the nucleus thanks to a powerful force known as the strong nuclear force. This mighty force overwhelms the electrostatic repulsion between protons, holding the nucleus together like an invisible magnet.
Without the strong nuclear force, the positively charged protons would fly apart, breaking the nucleus into its constituent parts. It's this delicate balance between electrostatic repulsion and the strong force that maintains the stability of the nucleus.
Type II: Neutron
- Define a neutron as a neutral particle in the nucleus.
- Explain the role of neutrons in providing stability to the nucleus.
- Discuss the variation in neutron count among isotopes of the same element.
Type II: The Enigmatic Neutron
In the heart of every atom lies an enigmatic particle that plays a crucial role in nuclear stability: the neutron. A neutral entity, the neutron doesn't carry any electrical charge, setting it apart from its positively charged counterpart, the proton.
The stabilizing role of the neutron is akin to a cosmic dance within the nuclear core. Protons, with their positive charges, naturally repel each other, threatening to tear the nucleus apart. Enter the neutron, acting as a mediator. Its neutral nature allows it to snuggle between protons, weakening their repulsive forces.
This balancing act is key to nuclear stability. The ratio of neutrons to protons determines the stability of an atom's nucleus. Too few neutrons, and the nucleus may succumb to the electrostatic repulsion between protons, leading to a radioactive decay. Conversely, an excess of neutrons can make the nucleus unstable as well.
The number of neutrons in an atom's nucleus also gives rise to the concept of isotopes. Isotopes are atoms of the same element with identical numbers of protons but varying numbers of neutrons. This variation in neutron count alters the mass of the atom while leaving its chemical properties essentially unchanged.
The intricacies of neutron behavior hold immense significance for both the stability of matter and the field of nuclear science. They pave the way for ongoing research and applications, ranging from nuclear energy to medical imaging, unlocking the secrets of the atomic realm and shaping our understanding of the universe.
The Nucleus: Understanding the Core of the Atom
The nucleus, the central core of an atom, holds the key to some of the most fundamental forces and particles in the universe. Inside this tiny realm, protons and neutrons coexist, each playing a critical role in shaping the atomic properties and the very existence of matter.
The nucleus houses protons, the positively charged particles that determine an element's atomic number and identity. These subatomic building blocks hold the key to an element's chemical properties and its place on the periodic table. Protons are held together by the strong nuclear force, a powerful force that defies electrostatic repulsion – the tendency of positively charged particles to repel each other.
Balancing the protons are the neutrons, neutral particles that add mass to the nucleus without altering its charge. Neutrons are crucial for nuclear stability, providing additional strong nuclear force to overcome the electrostatic repulsion between protons. The number of neutrons in a nucleus varies, resulting in different isotopes of the same element.
Within the nucleus, electrostatic forces and strong nuclear forces engage in a delicate dance. The strong nuclear force keeps protons together, but the electrostatic force attempts to push them apart. This balance is crucial for the stability of the nucleus. If the strong nuclear force is too weak, the protons will fly apart; if it is too strong, the nucleus will collapse.
The interplay of protons and neutrons in the nucleus has profound implications for atomic properties. The number of protons and neutrons determines the atomic mass of an atom, while the neutron-to-proton ratio influences the stability of the nucleus and its susceptibility to radioactivity. These nuclear properties shape the behavior of elements in chemical reactions, determining their reactivity, bonding affinities, and overall characteristics.
Understanding the protons and neutrons within the nucleus is central to nuclear science. Ongoing research delves into the depths of nuclear physics, exploring the nature of strong nuclear force, the behavior of subatomic particles, and the potential for practical applications. From harnessing nuclear energy to advancing medical imaging, the exploration of nuclear particles continues to unlock new frontiers in science and technology.
Impact on Atomic Properties
Atomic properties are profoundly influenced by the interplay between protons and neutrons within the atomic nucleus. These particles play a crucial role in shaping the fundamental characteristics of elements.
The number of protons in an atom's nucleus determines its atomic number and thus its position on the periodic table. It dictates the elemental identity of the atom and influences its chemical behavior. The number of neutrons, on the other hand, does not affect the element's chemical properties but contributes significantly to its atomic mass. The greater the number of neutrons, the heavier the atom.
The neutron-to-proton ratio has a profound impact on nuclear stability and radioactivity. Isotopes, which are atoms of the same element with varying neutron counts, exhibit different stability profiles. Elements with an imbalanced neutron-to-proton ratio tend to be unstable and undergo radioactive decay to achieve a more stable configuration. The neutron-to-proton ratio also influences the likelihood of nuclear reactions, such as fission and fusion.
Finally, the nuclear properties of an atom contribute to its behavior in chemical reactions. Elements with a high neutron-to-proton ratio often have lower chemical reactivity, as the additional neutrons stabilize the nucleus and make it less susceptible to interactions with other atoms. Conversely, elements with a low neutron-to-proton ratio tend to be more reactive, as their nuclei are more prone to undergo changes. Understanding the nuclear properties of elements provides valuable insights into their chemical behavior and enables scientists to predict and control chemical reactions.
**Delving into the Nucleus: The Two Elemental Particles that Shape Our World**
The heart of every atom lies within its nucleus, a tiny, dense core that houses the building blocks of matter. Among these fundamental particles are two enigmatic individuals: the proton and the neutron. Their unique properties and dynamic interplay shape the very essence of elements and drive countless phenomena in our universe.
**The Mighty Proton: Positively Charged and Defining Identity**
Protons reside within the nucleus, each carrying a positive electrical charge. Their abundance determines an element's atomic number and consequently its position on the periodic table. Protons play a central role in maintaining the balance of forces within the nucleus, counteracting the electrostatic repulsion between its positively charged inhabitants.
**The Neutral Neutron: A Balancing Act for Nuclear Stability**
Unlike protons, neutrons carry no electrical charge. However, their presence in the nucleus is vital for atomic stability. Neutrons act as a bridge between protons, neutralizing their electrical repulsion and allowing for the formation of atomic nuclei with higher numbers of protons. The delicate balance between protons and neutrons governs the stability of isotopes, variations of the same element with different neutron counts.
**The Nucleus: A Battleground of Forces**
Within the nucleus, an intricate dance of forces unfolds. The strong nuclear force, an incredibly powerful attraction between protons and neutrons, overcomes the electrostatic repulsion between like-charged protons. This delicate equilibrium maintains the integrity of the nucleus, preventing it from disintegrating under the immense forces at play.
**Impact on Atomic Properties: Mass and Beyond**
The number of protons and neutrons within an atom influences its atomic mass. Additionally, the neutron-to-proton ratio has a profound impact on nuclear stability and the likelihood of radioactive decay. The interplay between these nuclear particles ultimately shapes the behavior of elements in chemical reactions and defines their unique characteristics.
**Current Research and Applications: Unlocking Nuclear Secrets**
Ongoing research in nuclear physics delves into the deepest mysteries of matter, exploring the behavior of protons and neutrons in extreme conditions. Their insights hold promise for advancements in nuclear energy, providing clean and sustainable sources of power. Understanding nuclear particles also aids in medical imaging techniques, such as PET scans, which rely on the interactions of radioactive isotopes with biological tissues.
As we continue to unravel the secrets of the nucleus, we gain a deeper appreciation for the fundamental building blocks of our world. The collaboration of protons and neutrons, their intricate dance of forces, and their impact on atomic properties shape the very nature of matter and the wonders of our universe.
Related Topics:
- Engage Audiences, Build Credibility, And Inspire Action: Choosing Captivating Informative Speech Topics
- Master Intermediate Algebra: Unlock Advanced Mathematical Concepts For Success
- Unlock The Secrets Of Communicating With The Spirit Realm: A Comprehensive Guide To Mediumship, Ghost Hunting, And The Paranormal
- How To Cook Perfectly Seasoned Beans: A Comprehensive Guide For Beginners
- Comprehensive Guide To Distance, Travel, And Cultural Nuances Between China And Japan